Facts about Lime
* One ton of manufactured cement (1400–1450 °C) releases 850kg of carbon dioxide into the atmosphere ! This is a consequence of the chemical reaction and cannot be reduced by energy saving.
* The use of lime instead of cement will save approximately 80% of the CO2 release compared to ordinary cement. One single residence will save between 5,000 and 10,000 lbs of CO2 emissions.
Download the History of Lime translation here (172kb)
Each year in the US alone, environmentally conscientious builders are saving several millions of pounds of CO2 release by simply avoiding the use of cement-based products, and choosing Natural Hydraulic Lime instead.
Lime Cycle
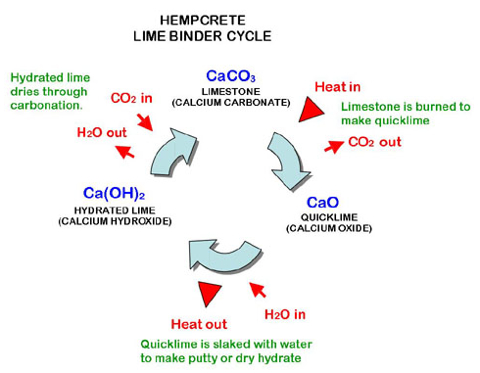
Note: CaCo3 (limestone) goes the full circle from being broken down to carbonation when Hempcrete is formed. As the Hempcrete hardens from carbonation, it will eventually petrify the hemp and form limestone.
It’s the lime that makes Hempcrete so special. When lime exists in nature, in the limestone, it is calcium carbonate — CaCO3 (an atom of calcium, carbon, and three oxygen atoms). Fire that up to around 900 degrees Celsius and the carbon dioxide — CO2 (an atom of carbon and two of oxygen) — burns off. That leaves you with calcium oxide, or "quicklime" — CaO (an atom of calcium and oxygen). Add a small amount of water (H2O) - two atoms of hydrogen and an atom of oxygen — and you get the powdery hydrated lime needed for Hempcrete, wholla
Download how lime is made here (1.1mb)

To make lime plaster, Limestone (calcium carbonate) is heated to produce quicklime (calcium oxide). Water is then added to produce slaked lime (calcium hydroxide), which is sold as a white powder. Additional water is added to form a paste prior to use. The paste may be stored in air-tight containers. Once exposed to the atmosphere, the calcium hydroxide turns back into Limestone, causing the plaster to set.
Lime plasters are used for true frescoes. Pigments, diluted in water, are applied to the still wet plaster. Modern stucco, which incorporates Portland cement, is also referred to as Lime Plaster, but this is incorrect.
Lime Mortar

Hydrated Lime
Hydrated lime can ONLY set through carbonation (re-absorption of CO2). It is often sold in builder's merchants as 'bagged' or hydrated “S” or “N” lime or is available as lime putty (or as quicklime to be made into lime putty), lime putty generally being considered more suitable for pure lime application. Hydrated lime is the most commonly used and known lime, also called (high) calcium lime or air lime, as it sets only by reaction with CO2 in the air and will not set until dry. This causes limitations in construction use as the lime can remain soft for months or years.
Hydraulic and Hydrated limes must not be confused:
Hydraulic Lime refers to a characteristic of the lime.
Safety issues
Lime is an extremely caustic material when wet, with a pH of 12. (Lime becomes pH neutral when carbonated). Protective goggles and gloves should be worn at all times. Additionally, protective clothing should be worn where risk of splatter on to bare skin is present.
Clean water should always be at arms length in case lime gets in someone’s eyes or on their skin. Skin can be neutralized with a very mild acid such as vinegar or lemon juice. Repeatedly flush eyes with fresh water for several minutes and consult medical advice.
Lime Wash
Unlike modern paints, which lay on the surface of the substrate, lime wash instead acts like a stain by penetrating deep into the pores of the underlying material. This process creates a peel-free, breathable surface, and the lime wash remains vapor permeable after it cures. It is a beautiful, traditional material that mellows while it gradually wears away, and over time it develops the weathered patina that characterizes the Old-World charm of Europe.
Use in architecture


Passive fire protection
Plasters have been in use as "passive" fireproofing products, for many decades: The finished plaster releases water vapor when exposed to flame, acting to slow the spread of the fire, for as much as an hour or two depending on thickness. It also provides some insulation to retard heat flow into structural steel elements, that would otherwise lose their strength and collapse in a fire.
For more information on calcium hydroxide click here for Wikipedia
